Designing the worlds first AI decentralised clinical trial (DCT) platform with eConsent and ePRO solutions.
My role
Head of UX and design, responsible for designing a modern clinical trials platform from 0-1 that is both powerful and easy to use. The platform allowed physicians to create trial recruitment landing pages, build consent forms, prescreen and recruit patients, collect data and analysing results.
To create such a platform, a blend of creativity, empathy, and technical expertise was required to make it a reality.
With my team, I lead the discovery research, user interviews, wireframes, user testing, designs and refinement & iterations of the eConsent product.
Product release date June 2022
Highlights
- 63% increase in revenue
- 98% CSAT from 3 million patients
- Increased users from 57,000 to 85,000
- Castors fastest growing product
- Designed 5 products across web & mobile
- Scaling 3X design team growth
- Raised a $45 million series B
The Challenge
Clinical trials last between 15-25 years due to poor patient recruitment, site costs, study design and insufficient data. How can we reduce this to 10 years by removing physical sites ($20-50k per site), paper processes and easier patient recruitment by giving global access – all whilst remaining regulatory and HIPAA compliant.
Castor had a decades old CDMS (Clinical Data Management System) with lacklustre features years behind its competitors. To gain traction with SMB and enterprise clients we needed to improve the current CDMS and create a brand new eConsent product.
Research & user interviews – how we knew it was a problem worth solving
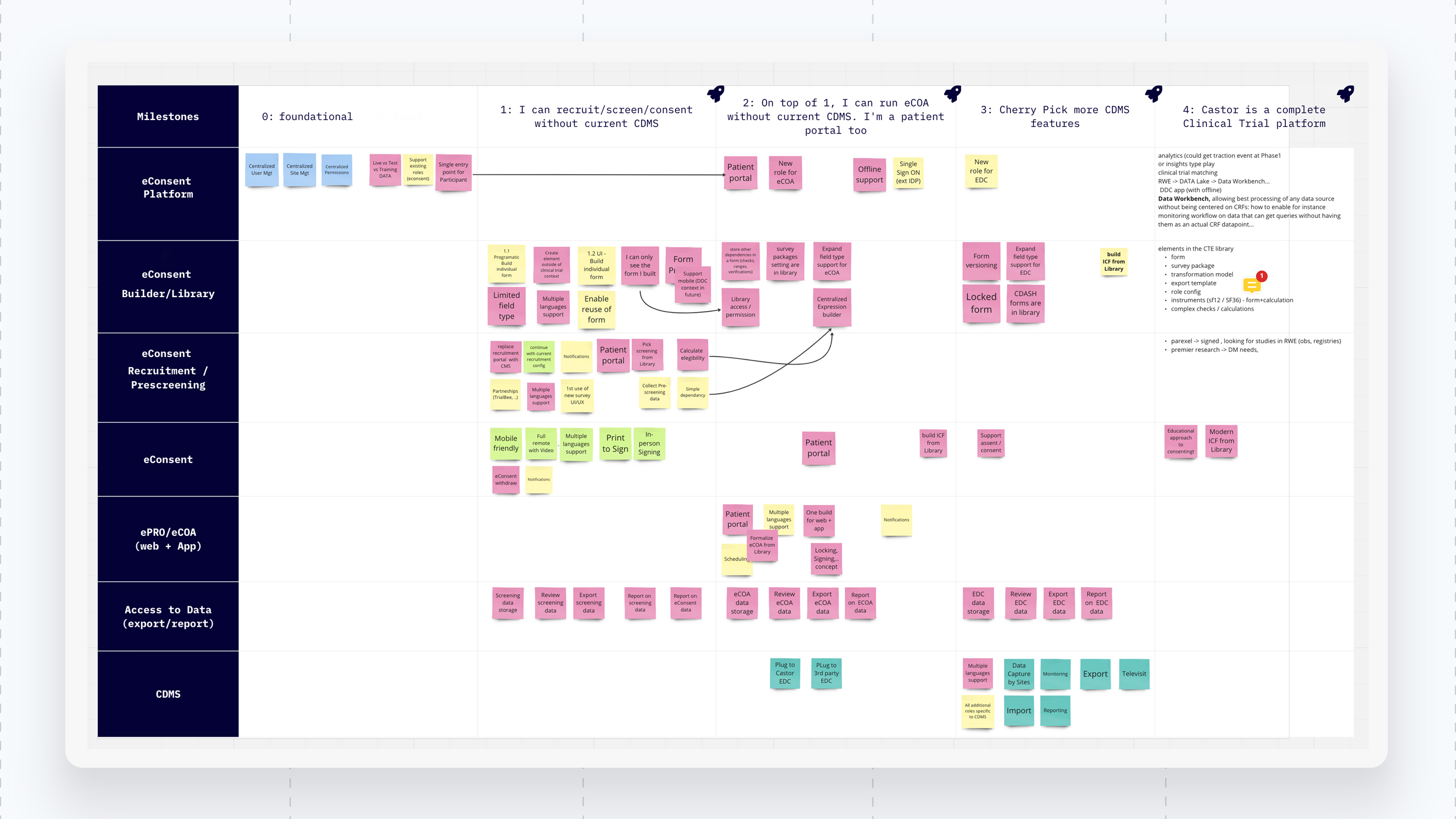
Interviewing physicians, patients and SME’s, I story mapped and created multiple user journeys looking at pain points, touch points and opportunities to improve processes. From the discovery phase I built up a big picture and defined the problems and priority mapped features to a roadmap. Ultimately, features were aligned to deal size and potential revenue generated by SMB and enterprise clients. This led for a clear need of a patient centric experience through an eConsent (Electronic Consent) and ePRO (Electronic Patient Reported Outcome) solution that integrates into the existing CDMS.
In the user research stage we interviewed 20 physicians, 8 patients in order for us to have a clear picture of the problem space. The picture below shows a snippet of many Miro boards I created – unfortunately I no longer have access to the boards and did not make copies for my portfolio.
Wireframes and user testing – Who we worked with and how
SME’s gave the initial direction to begin discovery and our users gave us the deeper contextual understanding to ideate possible solutions. We held regular design thinking workshops, usability and validation sessions with users, reporting findings to the wider business.
Every feature was wireframed and user tested with the 20 physicians, 8 patients for invaluable feedback on their expectations and preferences – this helped iterate on the solution before going into the final design. This user-centric approach was crucial in ensuring that the platform would resonate with its target audience and meet their needs.
Aligned with the product team, we could release final validated designs under feature flags and continue to measure results for further iteration. All the time engineering and QA leads were kept informed for technical feasibility and story point effort scoring.
I used the double diamond Product/UX process of iterative improvement and the RACI matrix to communication across departments and stakeholders, aligning everyone on a feature status. Quantitative, qualitative research, usability testing, user journeys, story mapping, wireframes, prototypes and validation testing with end users were all carried out by myself and my team.
The solution
We needed to do three essential things in order to create the optimal physician and patient workflow, launch the product from 0-1.
1. Design system
Allowing us to build faster and consistently over different experiences, building trust and confidence in our software.
2. eConsent platform
For physicians and study managers to create trials, recruit patients, schedule video calls and create consent forms.
3. ePRO solution
A user-centric platform for patients to participate and update progress in trials via SMS, a mobile app or a website.
1. Design system
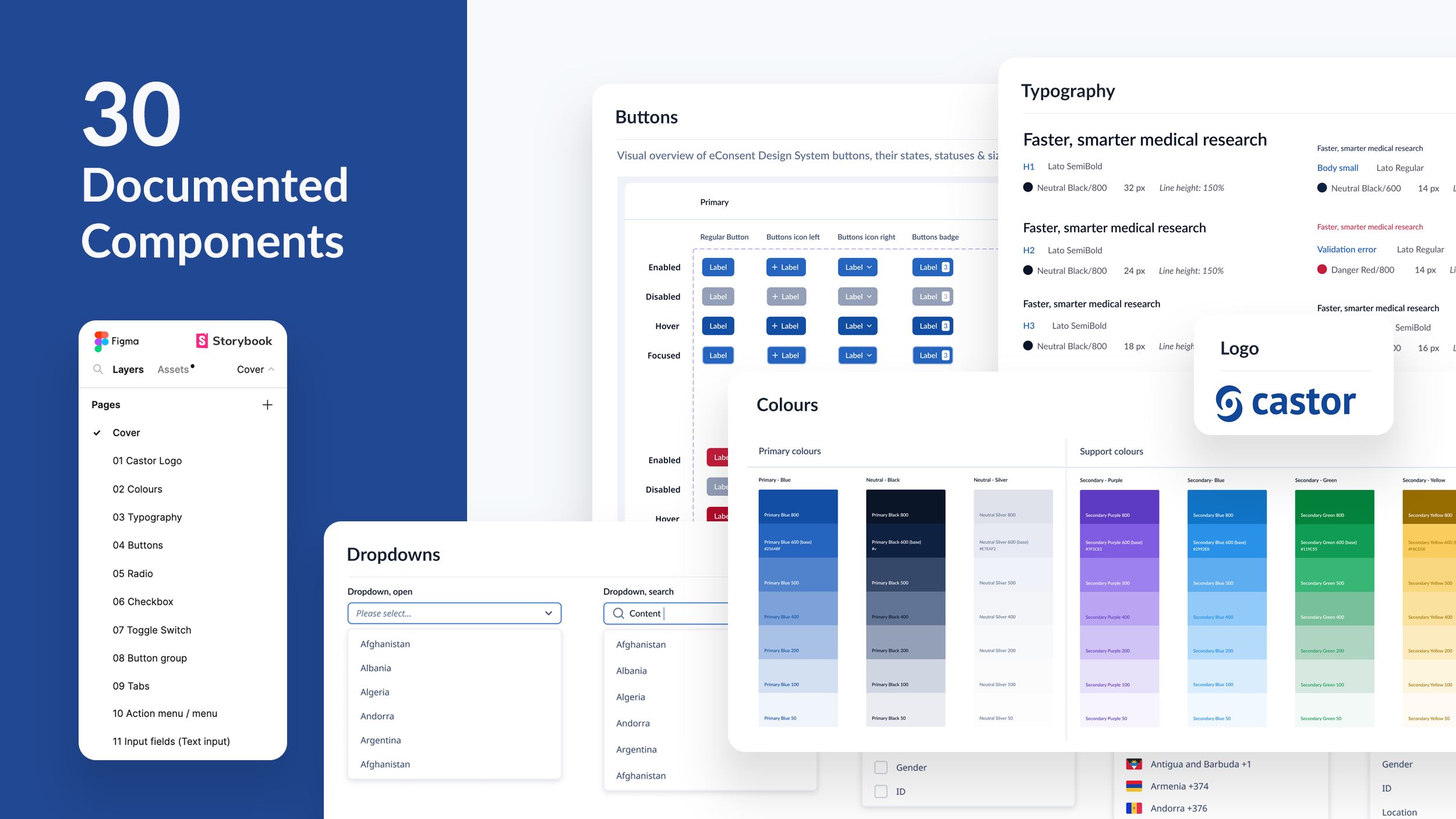
We created a documented design system (tokenized) for 90 engineers with Figma and Storybook integrations. This made feature design “drag and drop”, allowing us to deliver faster and focus on user experience over pixels. The consistent use of the design system across platforms reduced design, engineering and QA debt and resource dramatically, taking days/weeks instead of months to build features.
2. eConsent platform
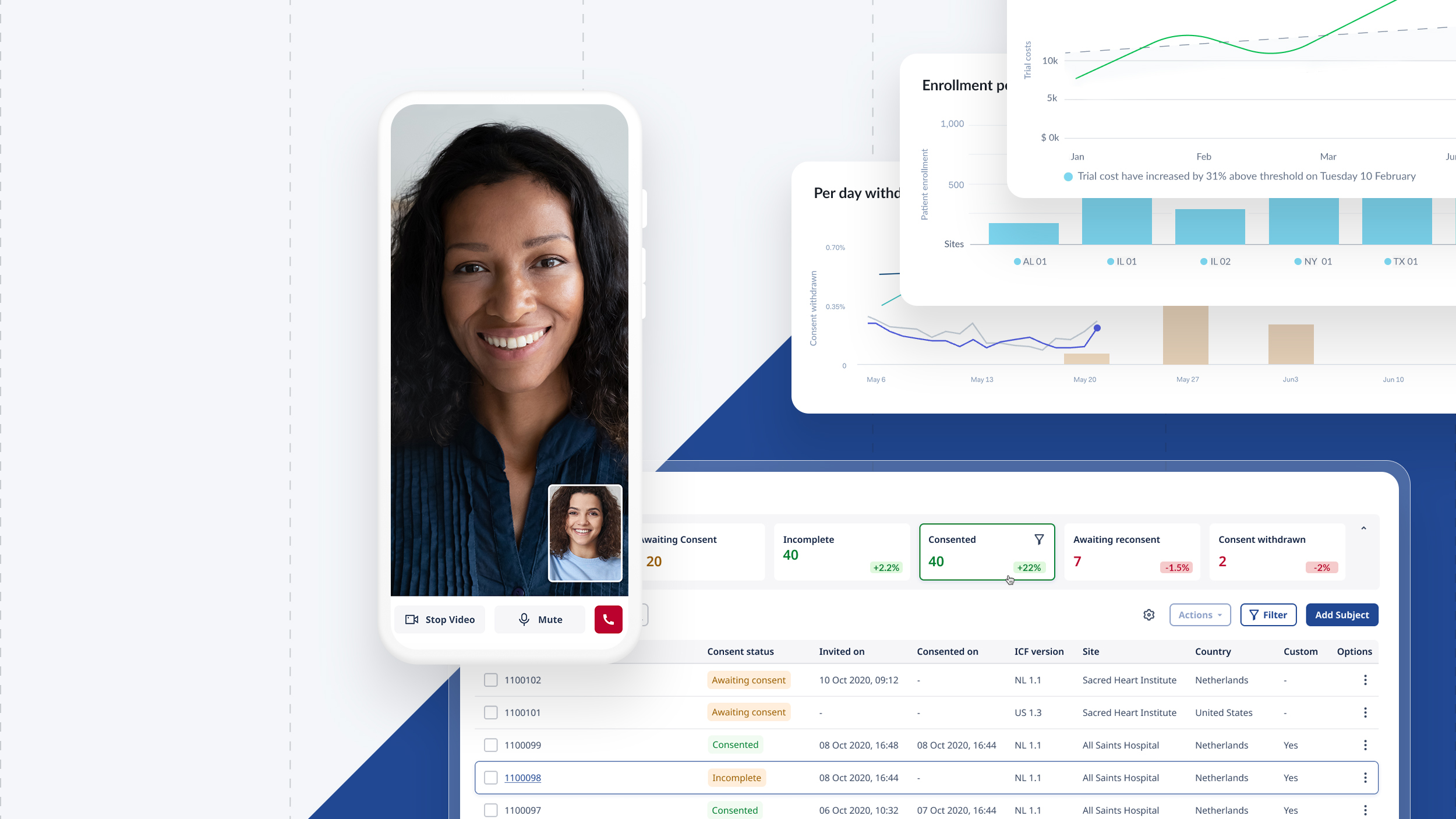
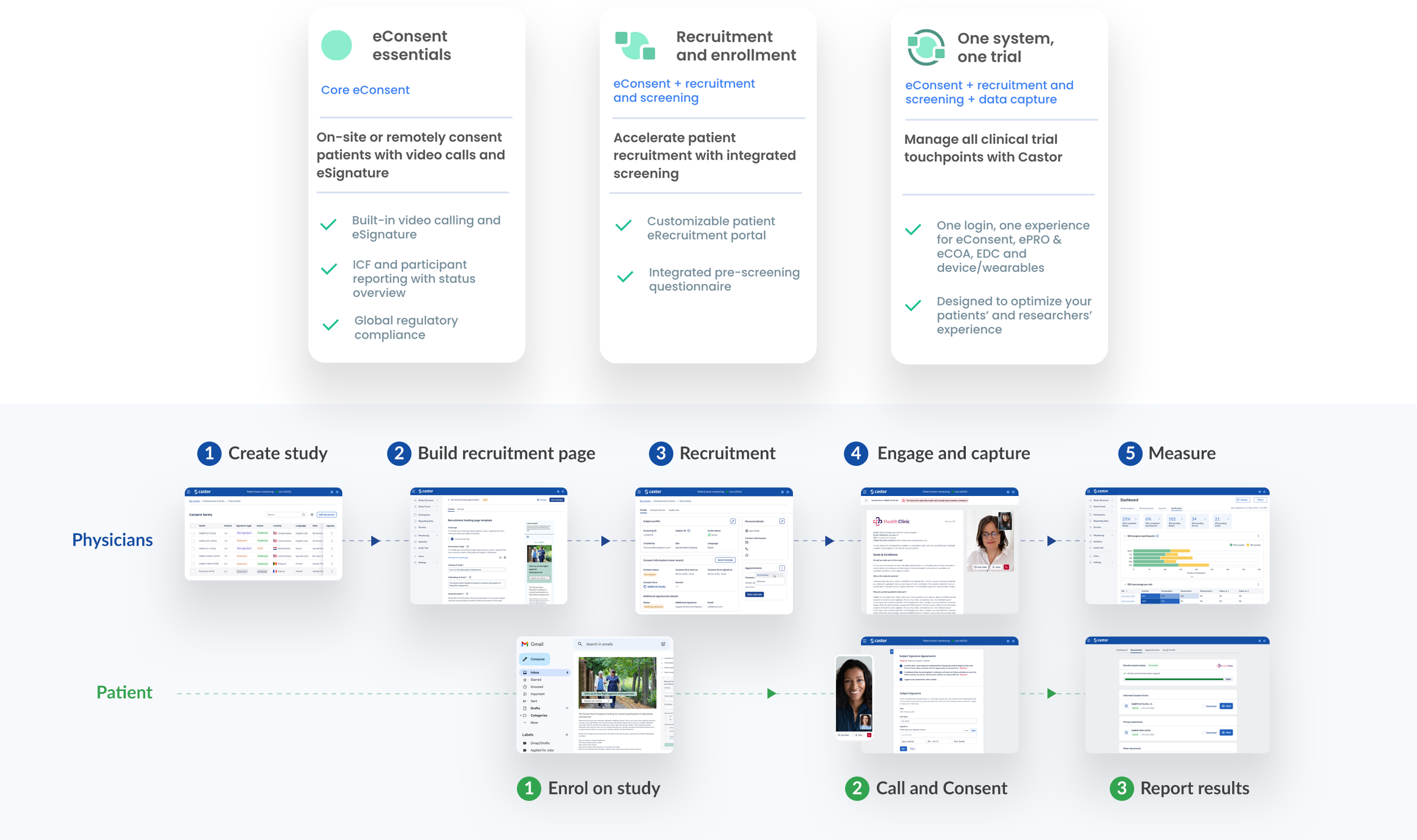
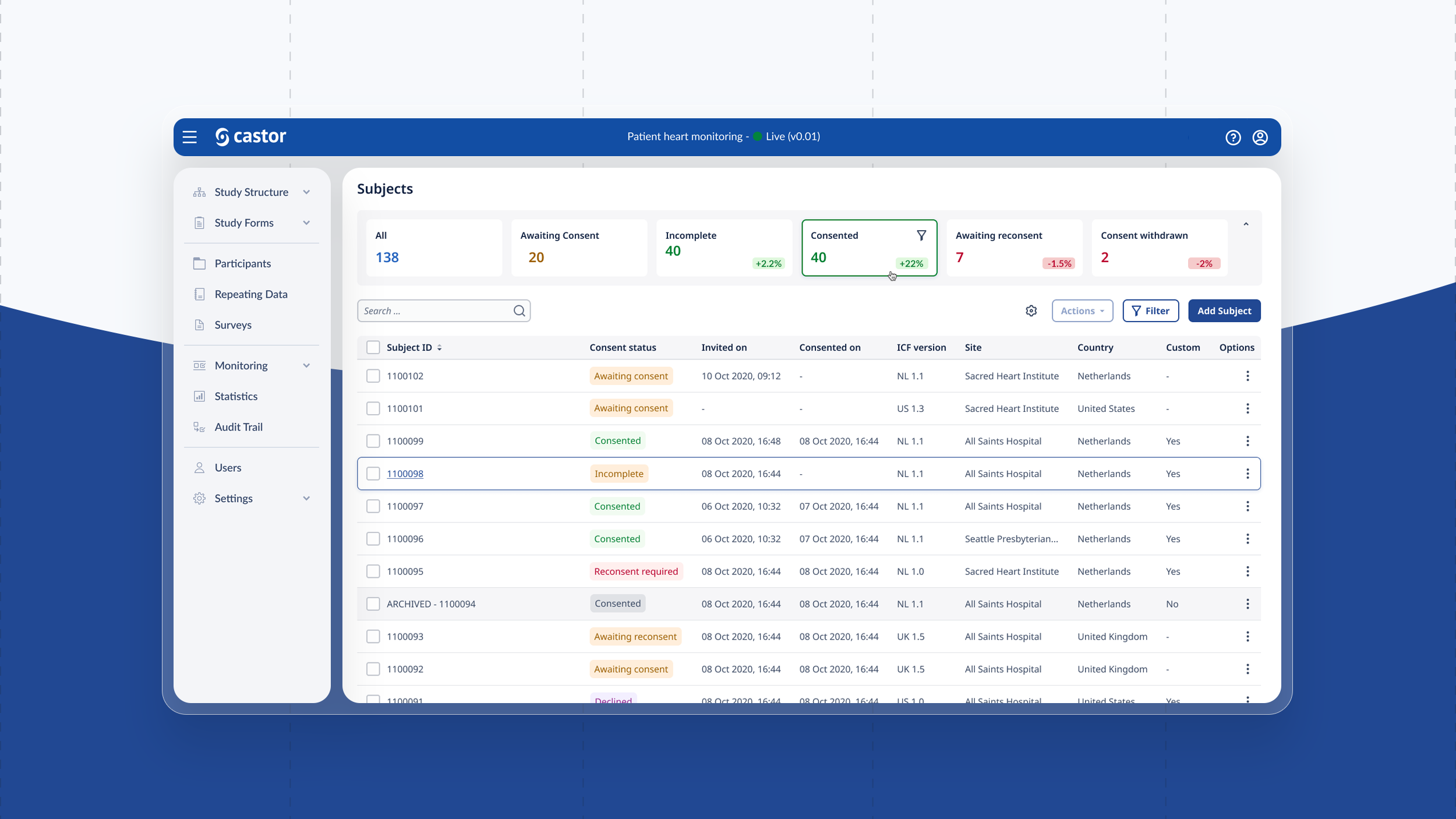
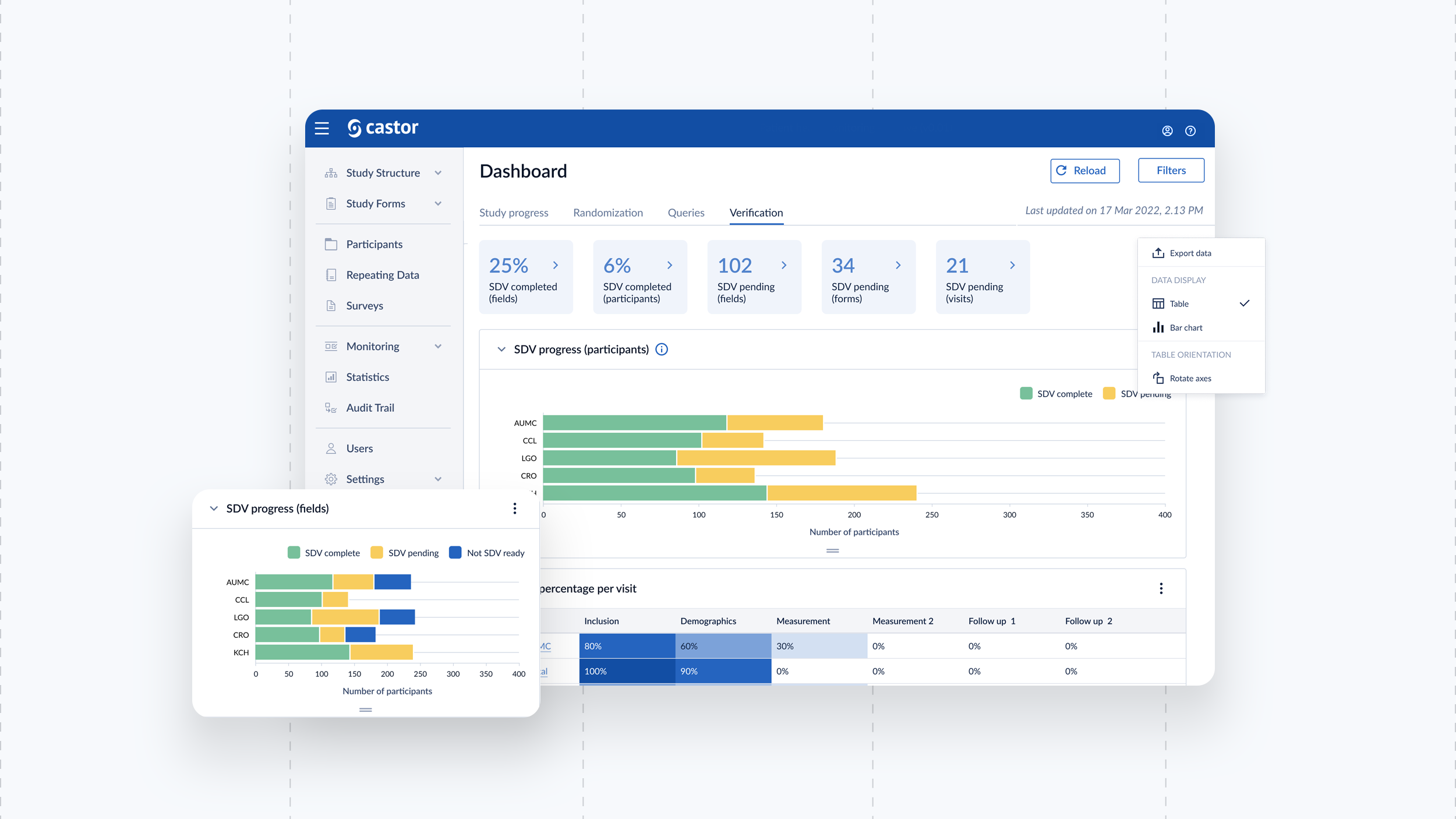
We built a brand new responsive eConsent platform for physicians and patients to participate effortlessly in trials with a global reach. From creating a database, building recruitment pages, mass emailing patients, creating consent forms, scheduling calls and reporting on data was possible from within the platform.
Physician & study manager features:
SSO (single sign on), Roles & permissions, Dashboards, Automations, Study builder, Survey form builder, Template management, Localisation, DocuSign integration, Document management, Call scheduling, Video calling televisits, Commenting / Chat
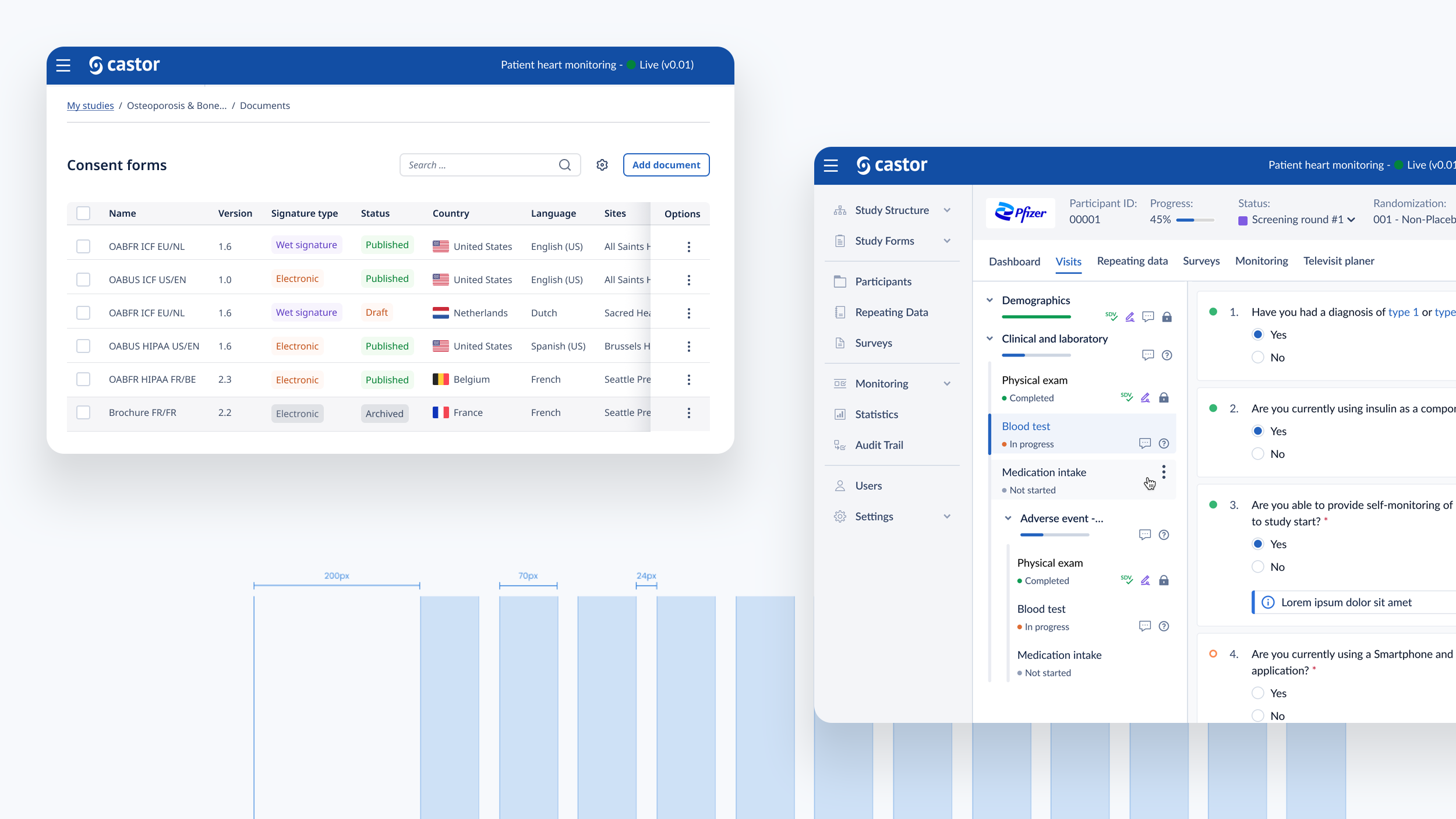
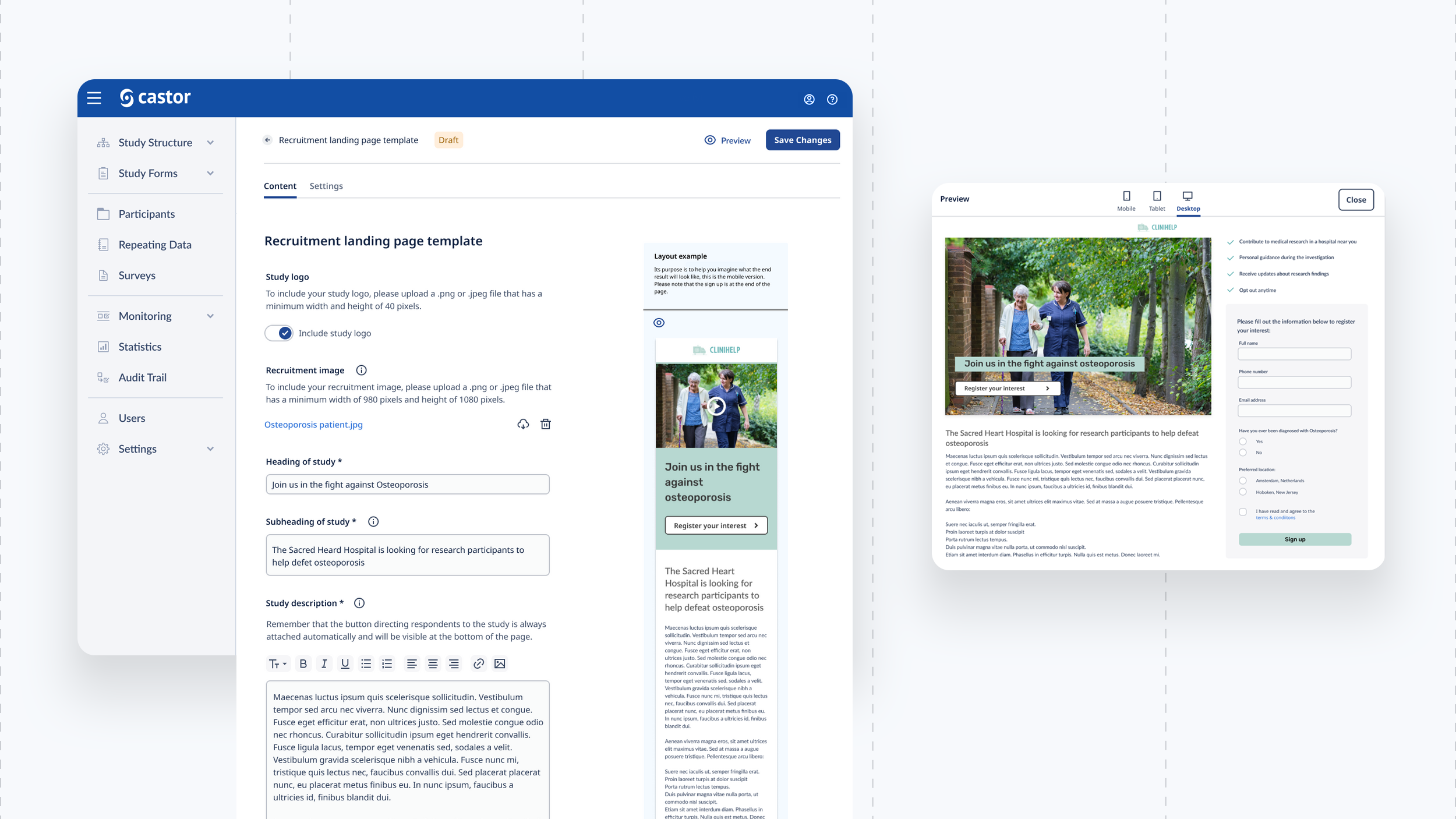
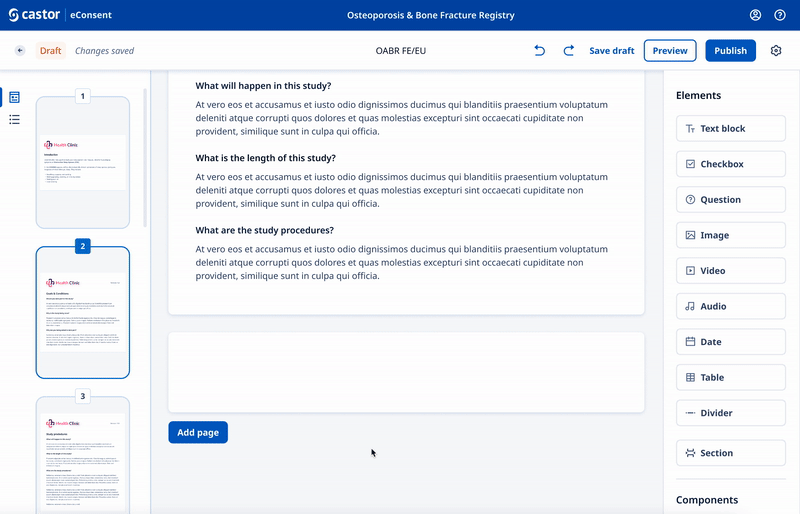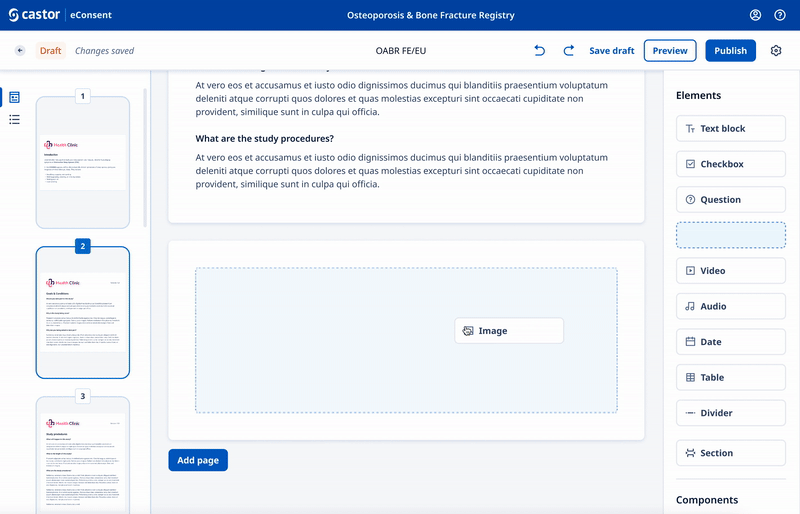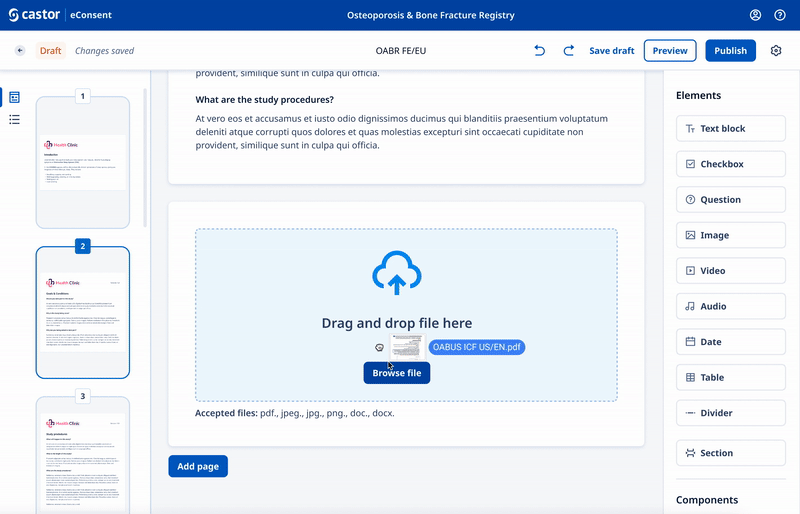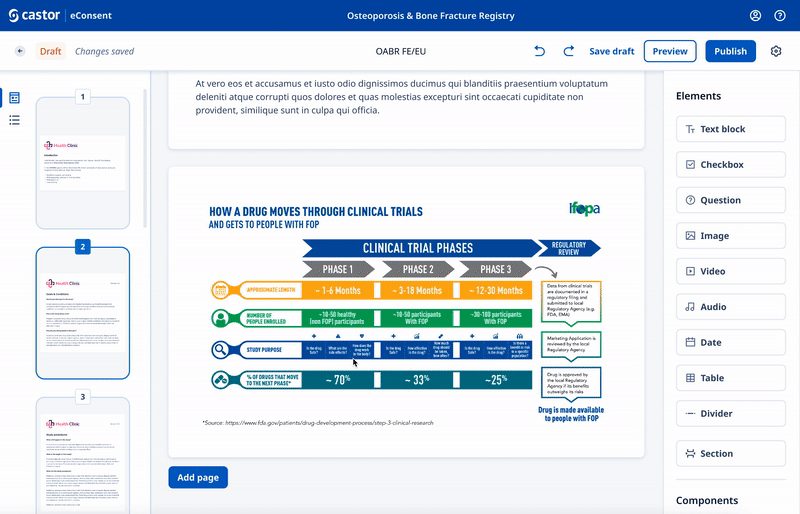
We designed a drag and drop document builder, prototyped in Figma for user testing before going into development.
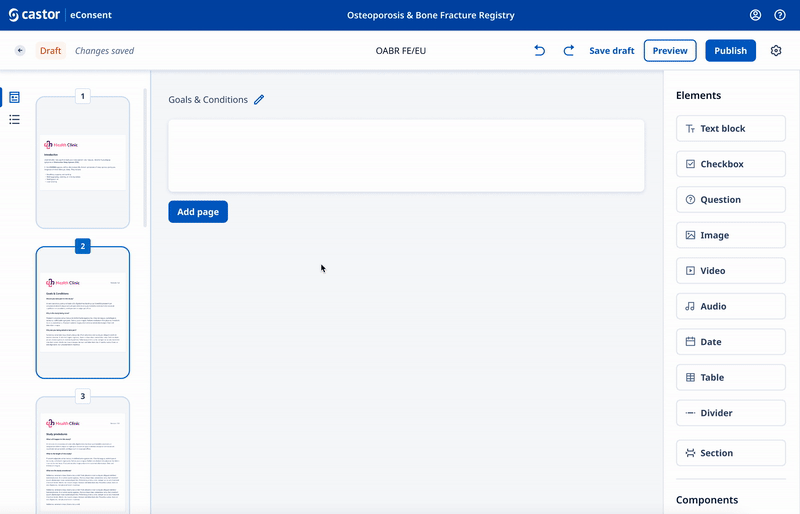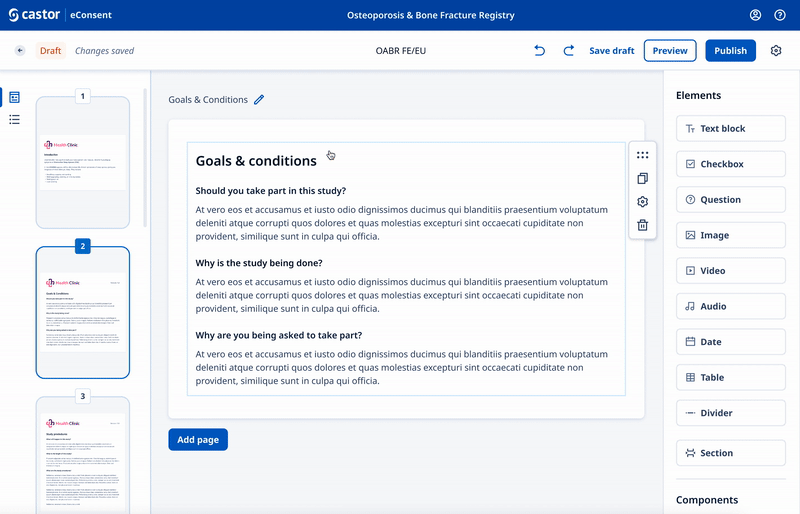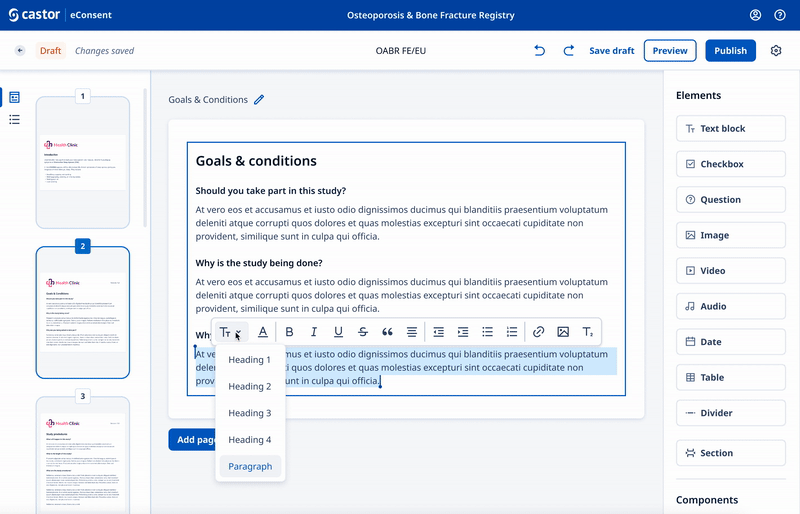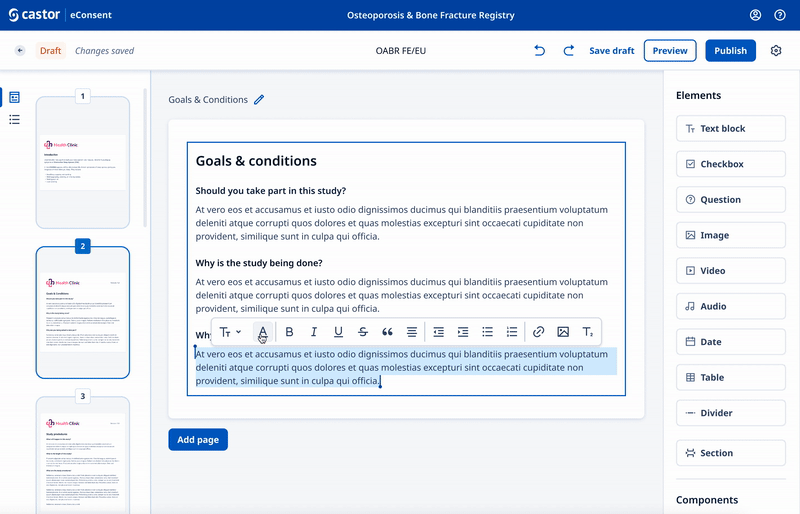
We experimented with 3rd party WYSIWYG editors to save development time and give ultimate flexibility.
Patient features:
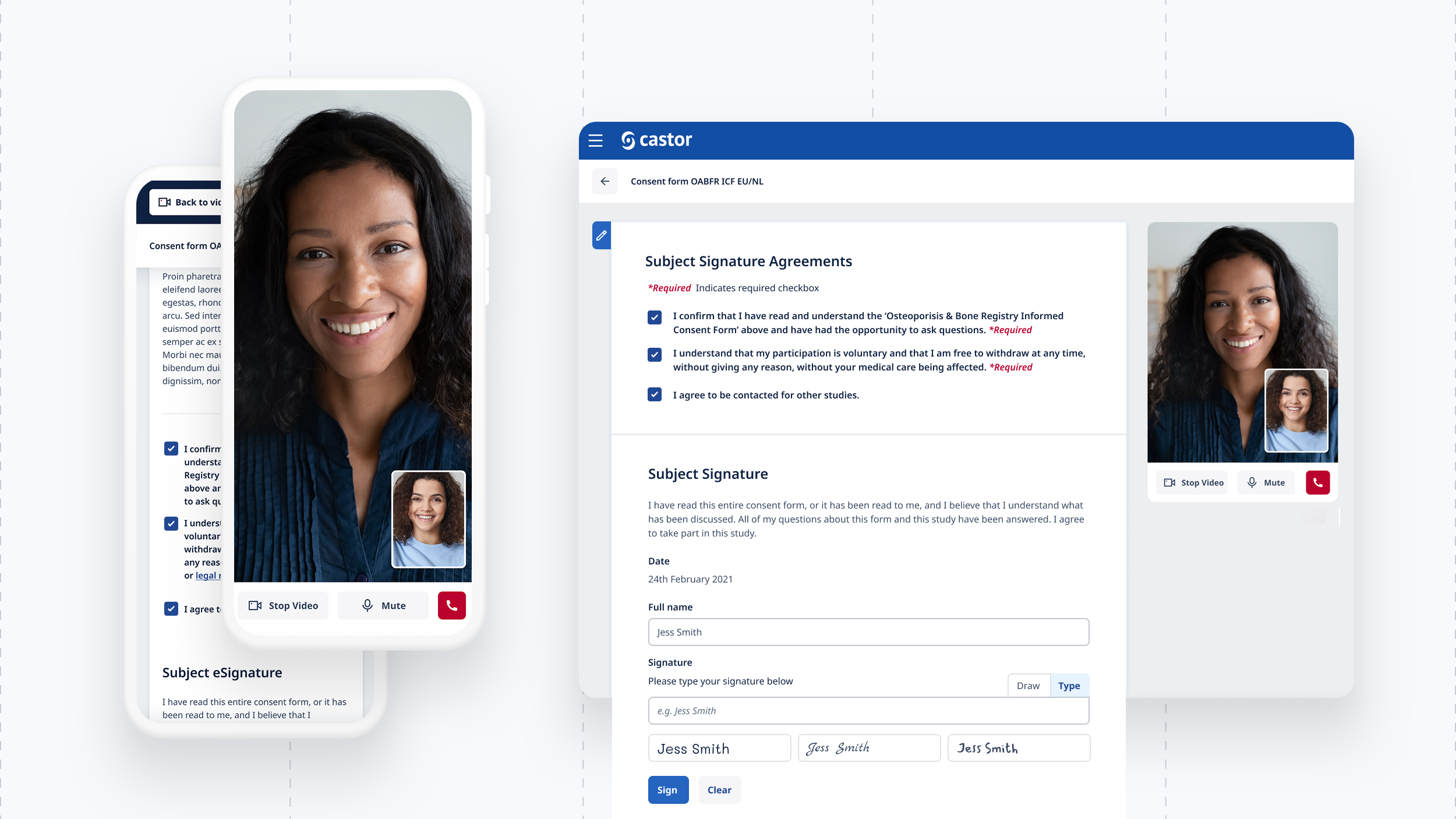
Document reviews, electronic signatures, commenting / chat, video calls, terminology query with Chat GPT and personal privacy settings.
Patients are invited to a study and if successfully pass onboarding, would be emailed/text a portal link. They would be welcomed to a personal study dashboard. From the dashboard they see all documents, appointments and the study profile including control over how personal information is used and shared.
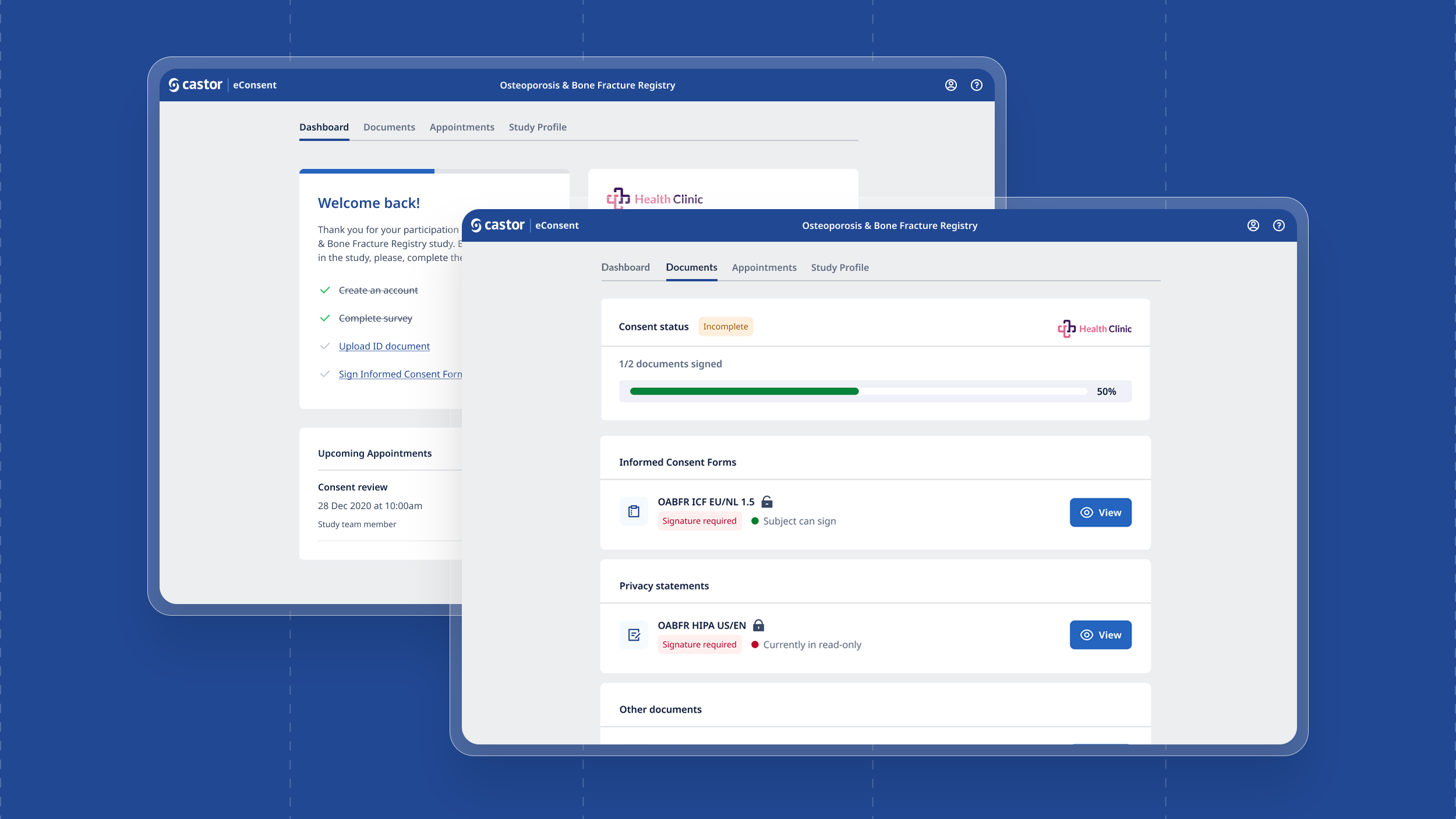
3. ePRO (Electronic Patient Reported Outcome)
Physicians use the new eConsent study and form builder to send surveys to patients.
Patients receive notifications via text, email or on the mobile app to answer surveys for self reporting. Two ways of self reporting were chosen because not everyone has a mobile phone and not everyone has an email address.
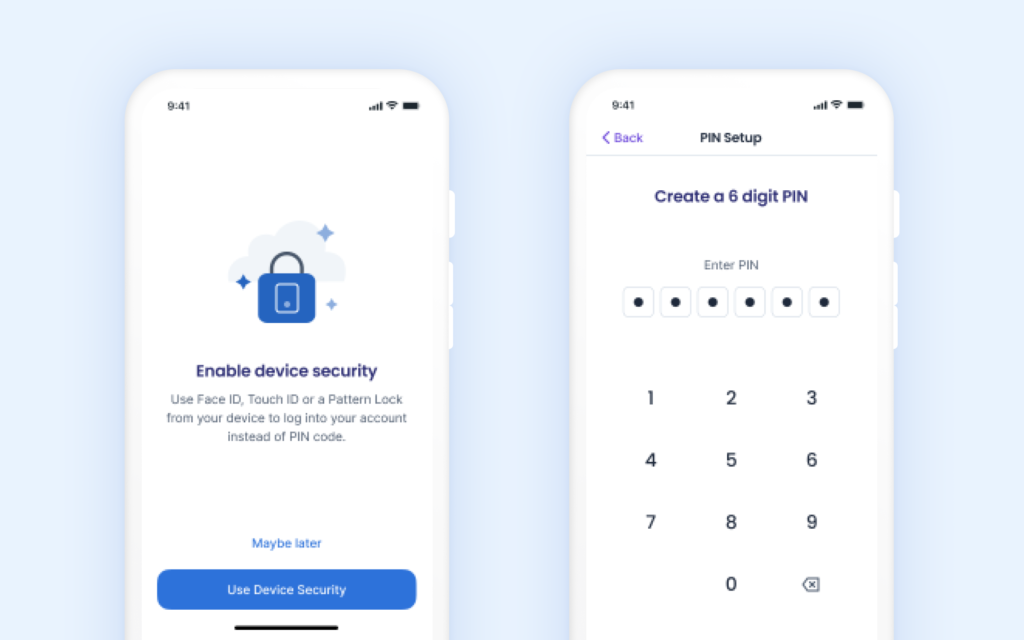
Enhanced security of patient data including two-factor authentication.
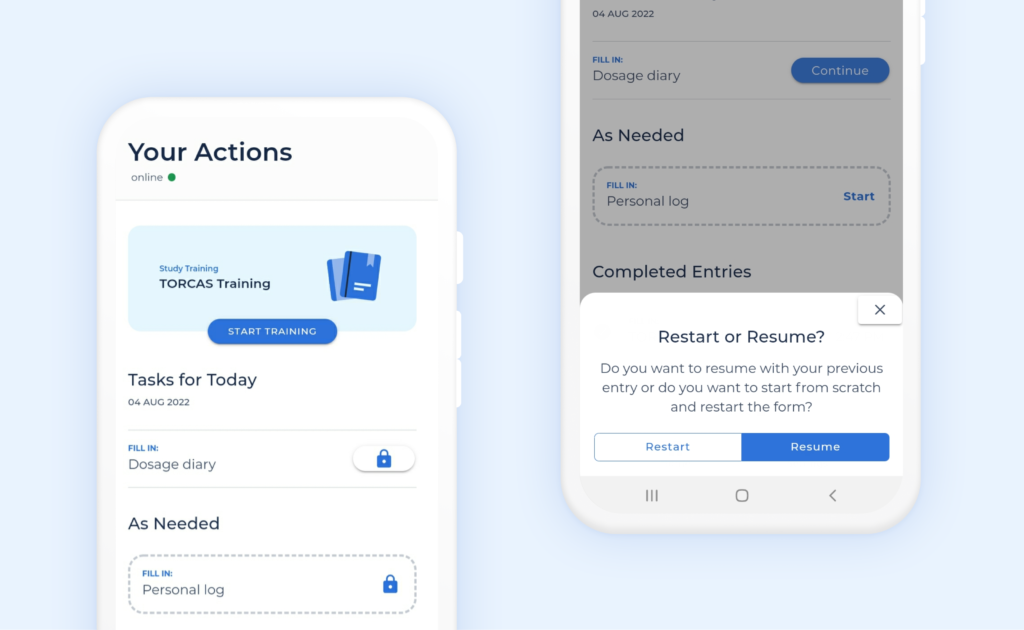
Patient self reporting mobile app on iOS and Android platforms.
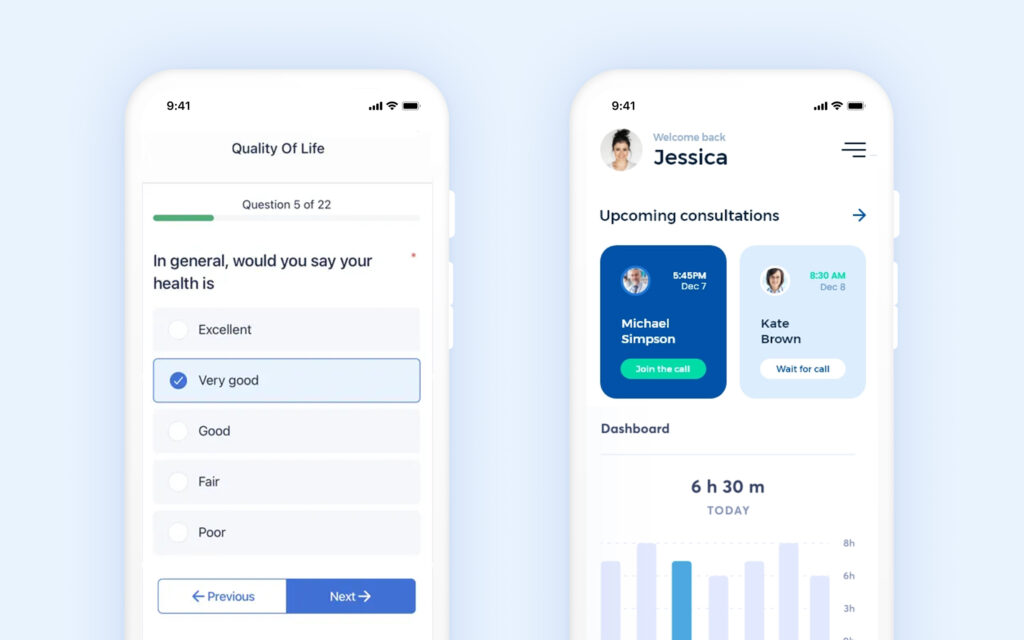
Patient self reporting surveys and consultation schedules.
The result
This project was a testament to the power of passion, collaboration, and user-centric design. By focusing on understanding and addressing the pain points and challenges faced by our users, we were able to create a platform that truly catered to their needs.
When leaving Castor, I had designed and validated several new features 9 months ahead of the product roadmap. This leaves a great path forward for the product and engineering teams to continue after I’m gone.